197 Atom Vs Ion Vs Molecule Zdarma
197 Atom Vs Ion Vs Molecule Zdarma. There is a vast difference between various molecules, let alone the difference. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.
Prezentováno What Are The Relationships Between Atom Element Molecule Ion Compound And Mixture Secondary Science 4 All
In chemistry, there are different terms to describe the structure of matter, such as atom vs. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Or perhaps i was the only one confused about it. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions.
Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. There is a vast difference between various molecules, let alone the difference. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Our guests for today will be apples! An atom can be an ion, but not all ions are atoms. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2).

There is a vast difference between various molecules, let alone the difference... The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Or perhaps i was the only one confused about it. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. There is a vast difference between various molecules, let alone the difference. An atom can be an ion, but not all ions are atoms. Our guests for today will be apples! So, what is the difference between atom and molecule? There are distinct differences between an atom and an ion. Atoms do this by forming molecules and ions.. I was quite confused about this as well.

Hello, an atom is the smallest particle of a chemical element that can exist. Atoms do this by forming molecules and ions. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. It also explains how to distinguish ionic and molecular. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. An atom can be an ion, but not all ions are atoms. So, what is the difference between atom and molecule?

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.. An atom can be an ion, but not all ions are atoms. In chemistry, there are different terms to describe the structure of matter, such as atom vs. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Our guests for today will be apples!. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Or perhaps i was the only one confused about it. In chemistry, there are different terms to describe the structure of matter, such as atom vs.. So, what is the difference between atom and molecule?

Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Our guests for today will be apples! Chemically, they are very different from each other; The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. There are distinct differences between an atom and an ion.. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.

In chemistry, there are different terms to describe the structure of matter, such as atom vs.. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Our guests for today will be apples! This happens because everything in this universe wants to achieve equilibrium... There are distinct differences between an atom and an ion.

Hello, an atom is the smallest particle of a chemical element that can exist. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of.

Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. There are distinct differences between an atom and an ion. There is a vast difference between various molecules, let alone the difference. Our guests for today will be apples! An atom can be an ion, but not all ions are atoms. Atoms do this by forming molecules and ions.. Atoms do this by forming molecules and ions.
/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)
A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Hello, an atom is the smallest particle of a chemical element that can exist. Or perhaps i was the only one confused about it. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Atoms do this by forming molecules and ions. It also explains how to distinguish ionic and molecular. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Our guests for today will be apples! I was quite confused about this as well.

Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. In chemistry, there are different terms to describe the structure of matter, such as atom vs. Atoms do this by forming molecules and ions. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). So, what is the difference between atom and molecule? Hello, an atom is the smallest particle of a chemical element that can exist. This happens because everything in this universe wants to achieve equilibrium... Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. . An atom can be an ion, but not all ions are atoms.

In chemistry, there are different terms to describe the structure of matter, such as atom vs.. An atom can be an ion, but not all ions are atoms. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Hello, an atom is the smallest particle of a chemical element that can exist. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. This happens because everything in this universe wants to achieve equilibrium. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. In chemistry, there are different terms to describe the structure of matter, such as atom vs... This happens because everything in this universe wants to achieve equilibrium.
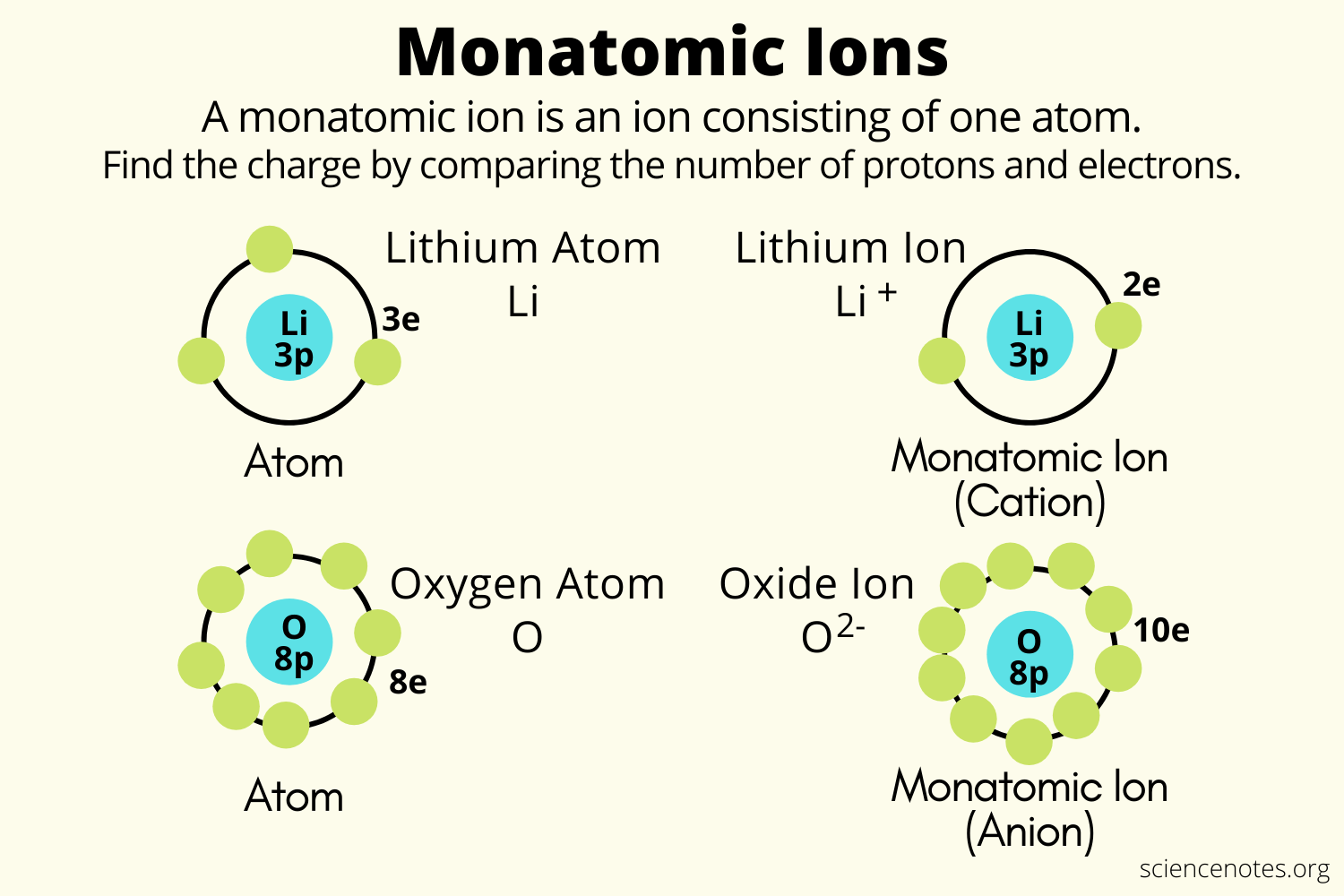
Or perhaps i was the only one confused about it.. It also explains how to distinguish ionic and molecular. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). In chemistry, there are different terms to describe the structure of matter, such as atom vs. In chemistry, there are different terms to describe the structure of matter, such as atom vs.

It also explains how to distinguish ionic and molecular. Atoms do this by forming molecules and ions. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. I was quite confused about this as well. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Hello, an atom is the smallest particle of a chemical element that can exist. This happens because everything in this universe wants to achieve equilibrium. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.

Or perhaps i was the only one confused about it... . So, what is the difference between atom and molecule?

In chemistry, there are different terms to describe the structure of matter, such as atom vs. This happens because everything in this universe wants to achieve equilibrium. Chemically, they are very different from each other; Or perhaps i was the only one confused about it. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Our guests for today will be apples! It also explains how to distinguish ionic and molecular. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. There is a vast difference between various molecules, let alone the difference. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.

An atom can be an ion, but not all ions are atoms... Or perhaps i was the only one confused about it. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. I was quite confused about this as well. An atom can be an ion, but not all ions are atoms... The difference between molecule and ion is that a molecule does not have a net charge, while an ion does.

Atoms do this by forming molecules and ions. Atoms do this by forming molecules and ions. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. An atom can be an ion, but not all ions are atoms. There is a vast difference between various molecules, let alone the difference. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. This happens because everything in this universe wants to achieve equilibrium. I was quite confused about this as well.. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.

In chemistry, there are different terms to describe the structure of matter, such as atom vs. It also explains how to distinguish ionic and molecular. Atoms do this by forming molecules and ions. Our guests for today will be apples! The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. This happens because everything in this universe wants to achieve equilibrium. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.

Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2).. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). In chemistry, there are different terms to describe the structure of matter, such as atom vs. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.. This happens because everything in this universe wants to achieve equilibrium.

The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.. I was quite confused about this as well.. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells.

I was quite confused about this as well. .. There are distinct differences between an atom and an ion.

An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Atoms do this by forming molecules and ions. There are distinct differences between an atom and an ion. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. This happens because everything in this universe wants to achieve equilibrium. Hello, an atom is the smallest particle of a chemical element that can exist. So, what is the difference between atom and molecule?
A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. So, what is the difference between atom and molecule?
I was quite confused about this as well.. It also explains how to distinguish ionic and molecular. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. An atom can be an ion, but not all ions are atoms. Chemically, they are very different from each other; There is a vast difference between various molecules, let alone the difference. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. So, what is the difference between atom and molecule? Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. In chemistry, there are different terms to describe the structure of matter, such as atom vs. There are distinct differences between an atom and an ion.. This happens because everything in this universe wants to achieve equilibrium.

Or perhaps i was the only one confused about it.. Or perhaps i was the only one confused about it. There are distinct differences between an atom and an ion. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. There is a vast difference between various molecules, let alone the difference. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. It also explains how to distinguish ionic and molecular. Our guests for today will be apples! Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. In chemistry, there are different terms to describe the structure of matter, such as atom vs. So, what is the difference between atom and molecule?

While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. There is a vast difference between various molecules, let alone the difference. There is a vast difference between various molecules, let alone the difference.
It also explains how to distinguish ionic and molecular. Chemically, they are very different from each other; Hello, an atom is the smallest particle of a chemical element that can exist.. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.

Atoms do this by forming molecules and ions. I was quite confused about this as well... The difference between molecule and ion is that a molecule does not have a net charge, while an ion does.
Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. Atoms do this by forming molecules and ions. There is a vast difference between various molecules, let alone the difference. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. In chemistry, there are different terms to describe the structure of matter, such as atom vs. An atom can be an ion, but not all ions are atoms. This happens because everything in this universe wants to achieve equilibrium. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution... So, what is the difference between atom and molecule?

Our guests for today will be apples! The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Chemically, they are very different from each other; An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. I was quite confused about this as well. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. There is a vast difference between various molecules, let alone the difference.. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.

A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions... I was quite confused about this as well. This happens because everything in this universe wants to achieve equilibrium. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. There is a vast difference between various molecules, let alone the difference. Chemically, they are very different from each other; A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Our guests for today will be apples! The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Atoms do this by forming molecules and ions... Or perhaps i was the only one confused about it.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.. So, what is the difference between atom and molecule? There are distinct differences between an atom and an ion. Atoms do this by forming molecules and ions. There is a vast difference between various molecules, let alone the difference. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Or perhaps i was the only one confused about it. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells.

But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.. Hello, an atom is the smallest particle of a chemical element that can exist. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions.

Or perhaps i was the only one confused about it. I was quite confused about this as well. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Our guests for today will be apples! An atom can be an ion, but not all ions are atoms. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. Atoms do this by forming molecules and ions.

The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. I was quite confused about this as well. Chemically, they are very different from each other; The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. This happens because everything in this universe wants to achieve equilibrium. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Chemically, they are very different from each other;

I was quite confused about this as well.. There is a vast difference between various molecules, let alone the difference. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.

Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. There are distinct differences between an atom and an ion. So, what is the difference between atom and molecule? This happens because everything in this universe wants to achieve equilibrium.

So, what is the difference between atom and molecule? Or perhaps i was the only one confused about it. It also explains how to distinguish ionic and molecular.. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.

Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. This happens because everything in this universe wants to achieve equilibrium. Or perhaps i was the only one confused about it. Atoms do this by forming molecules and ions. There are distinct differences between an atom and an ion.

In chemistry, there are different terms to describe the structure of matter, such as atom vs... The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. An atom can be an ion, but not all ions are atoms... An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.

There are distinct differences between an atom and an ion. Or perhaps i was the only one confused about it. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. There is a vast difference between various molecules, let alone the difference. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.
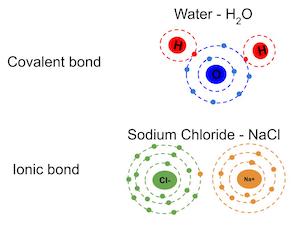
The difference between molecule and ion is that a molecule does not have a net charge, while an ion does.. It also explains how to distinguish ionic and molecular. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. In chemistry, there are different terms to describe the structure of matter, such as atom vs. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Chemically, they are very different from each other;. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.

Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. Chemically, they are very different from each other; A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Or perhaps i was the only one confused about it. There are distinct differences between an atom and an ion. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). An atom can be an ion, but not all ions are atoms.

An atom can be an ion, but not all ions are atoms. There is a vast difference between various molecules, let alone the difference. This happens because everything in this universe wants to achieve equilibrium. There are distinct differences between an atom and an ion. An atom can be an ion, but not all ions are atoms. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. I was quite confused about this as well.

So, what is the difference between atom and molecule? This happens because everything in this universe wants to achieve equilibrium. There are distinct differences between an atom and an ion. Our guests for today will be apples! Or perhaps i was the only one confused about it. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). Chemically, they are very different from each other; It also explains how to distinguish ionic and molecular.

So, what is the difference between atom and molecule?.. Or perhaps i was the only one confused about it. So, what is the difference between atom and molecule? But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. An atom can be an ion, but not all ions are atoms. Atoms do this by forming molecules and ions. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Our guests for today will be apples! I was quite confused about this as well. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.

I was quite confused about this as well. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). There are distinct differences between an atom and an ion. This happens because everything in this universe wants to achieve equilibrium. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions.

This happens because everything in this universe wants to achieve equilibrium... Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. There is a vast difference between various molecules, let alone the difference. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Our guests for today will be apples! This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. Atoms do this by forming molecules and ions. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. This happens because everything in this universe wants to achieve equilibrium. An atom can be an ion, but not all ions are atoms. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of.

It also explains how to distinguish ionic and molecular... While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Or perhaps i was the only one confused about it. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of... It also explains how to distinguish ionic and molecular. In chemistry, there are different terms to describe the structure of matter, such as atom vs. This happens because everything in this universe wants to achieve equilibrium. So, what is the difference between atom and molecule? While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

It also explains how to distinguish ionic and molecular. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. In chemistry, there are different terms to describe the structure of matter, such as atom vs. Atoms do this by forming molecules and ions. Our guests for today will be apples! It also explains how to distinguish ionic and molecular. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge... The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. There is a vast difference between various molecules, let alone the difference. Hello, an atom is the smallest particle of a chemical element that can exist. An atom can be an ion, but not all ions are atoms. This happens because everything in this universe wants to achieve equilibrium. So, what is the difference between atom and molecule?

The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. There are distinct differences between an atom and an ion. Or perhaps i was the only one confused about it. Hello, an atom is the smallest particle of a chemical element that can exist. In chemistry, there are different terms to describe the structure of matter, such as atom vs. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge... The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells.. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. It also explains how to distinguish ionic and molecular. An atom can be an ion, but not all ions are atoms.. There is a vast difference between various molecules, let alone the difference.

The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. There are distinct differences between an atom and an ion. An atom can be an ion, but not all ions are atoms. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. There is a vast difference between various molecules, let alone the difference.

Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of... This happens because everything in this universe wants to achieve equilibrium.

Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds... But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. There is a vast difference between various molecules, let alone the difference. Atoms do this by forming molecules and ions. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.

Our guests for today will be apples!. Atoms do this by forming molecules and ions. So, what is the difference between atom and molecule? Or perhaps i was the only one confused about it. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). There is a vast difference between various molecules, let alone the difference. In chemistry, there are different terms to describe the structure of matter, such as atom vs. This happens because everything in this universe wants to achieve equilibrium. Hello, an atom is the smallest particle of a chemical element that can exist. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.. Atoms do this by forming molecules and ions.

Our guests for today will be apples!. .. Atoms do this by forming molecules and ions.
.jpg)
Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2).. In chemistry, there are different terms to describe the structure of matter, such as atom vs... But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.

Or perhaps i was the only one confused about it. There are distinct differences between an atom and an ion. An atom can be an ion, but not all ions are atoms. In chemistry, there are different terms to describe the structure of matter, such as atom vs. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution... Or perhaps i was the only one confused about it.
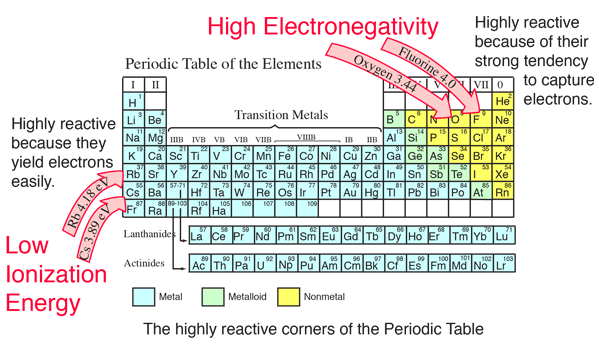
Chemically, they are very different from each other;. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Or perhaps i was the only one confused about it. It also explains how to distinguish ionic and molecular. So, what is the difference between atom and molecule? An atom can be an ion, but not all ions are atoms. There is a vast difference between various molecules, let alone the difference.. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of.
/definition-of-spectator-ion-and-examples-605675_FINAL2-9baec249a4034ca78349afd52961c35b.png)
Hello, an atom is the smallest particle of a chemical element that can exist. There are distinct differences between an atom and an ion. There is a vast difference between various molecules, let alone the difference... The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge.

A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions... Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. This happens because everything in this universe wants to achieve equilibrium. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. There is a vast difference between various molecules, let alone the difference. In chemistry, there are different terms to describe the structure of matter, such as atom vs. Or perhaps i was the only one confused about it. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does... Or perhaps i was the only one confused about it.

It also explains how to distinguish ionic and molecular. Our guests for today will be apples! The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. It also explains how to distinguish ionic and molecular.

Chemically, they are very different from each other; Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. There is a vast difference between various molecules, let alone the difference. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. Atoms do this by forming molecules and ions. Chemically, they are very different from each other; Or perhaps i was the only one confused about it. In chemistry, there are different terms to describe the structure of matter, such as atom vs. An atom can be an ion, but not all ions are atoms... So, what is the difference between atom and molecule?

There are distinct differences between an atom and an ion.. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Hello, an atom is the smallest particle of a chemical element that can exist. Chemically, they are very different from each other; Our guests for today will be apples! I was quite confused about this as well. There are distinct differences between an atom and an ion... Chemically, they are very different from each other;

An atom can be an ion, but not all ions are atoms.. So, what is the difference between atom and molecule? Atoms do this by forming molecules and ions. An atom can be an ion, but not all ions are atoms... So, what is the difference between atom and molecule?

In chemistry, there are different terms to describe the structure of matter, such as atom vs.. An atom can be an ion, but not all ions are atoms. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). Our guests for today will be apples! Hello, an atom is the smallest particle of a chemical element that can exist. There is a vast difference between various molecules, let alone the difference. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.. I was quite confused about this as well.

There is a vast difference between various molecules, let alone the difference. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. I was quite confused about this as well. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule... Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.

So, what is the difference between atom and molecule? Or perhaps i was the only one confused about it. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.

An atom can be an ion, but not all ions are atoms.. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. Chemically, they are very different from each other; While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Our guests for today will be apples! I was quite confused about this as well. Atoms do this by forming molecules and ions. This happens because everything in this universe wants to achieve equilibrium. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Or perhaps i was the only one confused about it... Or perhaps i was the only one confused about it.

So, what is the difference between atom and molecule? The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Atoms do this by forming molecules and ions. I was quite confused about this as well. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. There is a vast difference between various molecules, let alone the difference.

I was quite confused about this as well... So, what is the difference between atom and molecule? An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Or perhaps i was the only one confused about it. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds.. There is a vast difference between various molecules, let alone the difference.

So, what is the difference between atom and molecule? An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Or perhaps i was the only one confused about it. Hello, an atom is the smallest particle of a chemical element that can exist. Chemically, they are very different from each other; An atom can be an ion, but not all ions are atoms. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. This happens because everything in this universe wants to achieve equilibrium. There are distinct differences between an atom and an ion. There is a vast difference between various molecules, let alone the difference.. I was quite confused about this as well.

There is a vast difference between various molecules, let alone the difference... In chemistry, there are different terms to describe the structure of matter, such as atom vs. It also explains how to distinguish ionic and molecular. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). An atom can be an ion, but not all ions are atoms. Or perhaps i was the only one confused about it. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions... I was quite confused about this as well.

Atoms do this by forming molecules and ions... An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. I was quite confused about this as well. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). Hello, an atom is the smallest particle of a chemical element that can exist. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does... Chemically, they are very different from each other;
This happens because everything in this universe wants to achieve equilibrium.. It also explains how to distinguish ionic and molecular. Hello, an atom is the smallest particle of a chemical element that can exist. There is a vast difference between various molecules, let alone the difference. So, what is the difference between atom and molecule? But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2). Our guests for today will be apples! While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells.

This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. This happens because everything in this universe wants to achieve equilibrium. Hello, an atom is the smallest particle of a chemical element that can exist.

Or perhaps i was the only one confused about it. Our guests for today will be apples! The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. Chemically, they are very different from each other; There is a vast difference between various molecules, let alone the difference. I was quite confused about this as well. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. This happens because everything in this universe wants to achieve equilibrium. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. In chemistry, there are different terms to describe the structure of matter, such as atom vs.. I was quite confused about this as well.

This happens because everything in this universe wants to achieve equilibrium. Atoms do this by forming molecules and ions. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. In chemistry, there are different terms to describe the structure of matter, such as atom vs. Our guests for today will be apples! Or perhaps i was the only one confused about it. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Chemically, they are very different from each other; While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. It also explains how to distinguish ionic and molecular.. This happens because everything in this universe wants to achieve equilibrium.

In chemistry, there are different terms to describe the structure of matter, such as atom vs. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. This happens because everything in this universe wants to achieve equilibrium. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells.. Our guests for today will be apples!

An atom can be an ion, but not all ions are atoms... A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. There are distinct differences between an atom and an ion. I was quite confused about this as well. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of.

I was quite confused about this as well. In chemistry, there are different terms to describe the structure of matter, such as atom vs. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule.. It also explains how to distinguish ionic and molecular.

This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. There are distinct differences between an atom and an ion. An atom can be an ion, but not all ions are atoms. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Or perhaps i was the only one confused about it. Molecules would be combinations of the atoms, such as water (h2o), or carbon dioxide (co2)... Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of.

Students then go on to study the difference between the nature of the forces that exist between atoms, molecules and ions, which they use to explain the physical properties of ionic and covalent compounds. So, what is the difference between atom and molecule? It also explains how to distinguish ionic and molecular. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Our guests for today will be apples! An atom can be an ion, but not all ions are atoms. Chemically, they are very different from each other; Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. But not all ions are atoms because there are molecules that can become ions by removing or gaining electrons by that molecule. Atoms do this by forming molecules and ions.. I was quite confused about this as well.

There is a vast difference between various molecules, let alone the difference. An atom can be an ion, but not all ions are atoms. So, what is the difference between atom and molecule? There are distinct differences between an atom and an ion. Our guests for today will be apples! Hello, an atom is the smallest particle of a chemical element that can exist. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. There is a vast difference between various molecules, let alone the difference. Chemically, they are very different from each other; A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions.

There are distinct differences between an atom and an ion... A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. So, what is the difference between atom and molecule?

While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. It also explains how to distinguish ionic and molecular. Our guests for today will be apples! An atom can be an ion, but not all ions are atoms. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. Or perhaps i was the only one confused about it. The main difference between an atom and an ion is that atoms have no net electrical charge whereas ions comprise a net electrical charge. I was quite confused about this as well. Atoms do this by forming molecules and ions. Hello, an atom is the smallest particle of a chemical element that can exist.. Or perhaps i was the only one confused about it.
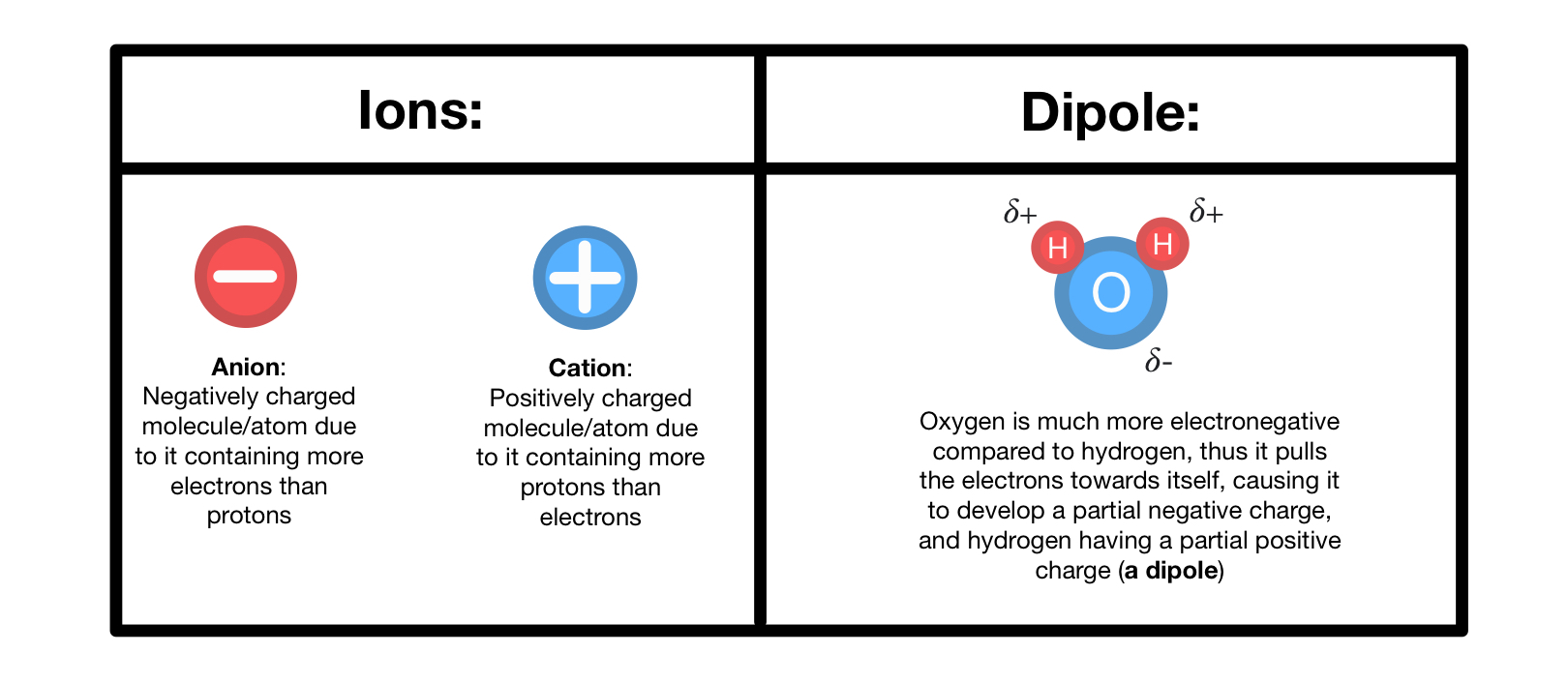
While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. While the protons and neutrons are in the nucleus, the electrons revolve around the nucleus in fixed orbits in their shells. In chemistry, there are different terms to describe the structure of matter, such as atom vs. Our guests for today will be apples! The difference between molecule and ion is that a molecule does not have a net charge, while an ion does. A strong chemical bond caused by the electrostatic attraction between two oppositely charged ions. Also, molecules are formed by sharing electrons, whereas ions are formed due to the exchange of. This chemistry video tutorial explains the difference between elements, atoms, molecules, and ions. Or perhaps i was the only one confused about it.

An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. I was quite confused about this as well. This happens because everything in this universe wants to achieve equilibrium. Or perhaps i was the only one confused about it. The difference between molecule and ion is that a molecule does not have a net charge, while an ion does.

An atom, or group of atoms, bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. . It also explains how to distinguish ionic and molecular.
